Addressing regulatory aspects in the development of remote measurement technologies for Alzheimer’s disease assessment
The Remote Assessment of Disease and Relapse – Alzheimer’s Disease (RADAR-AD) project, has made significant progress in utilizing remote measurement technologies (RMTs) to assess functional decline in the early stages of dementia. An important challenge encountered was navigating the regulatory landscape for the adoption of these novel technologies in clinical studies. Engagement with the European Medicines Agency (EMA) provided essential feedback to support this effort. The advisory process lasted 7 months, preceded by a 5-month period dedicated to compiling the Briefing Book, which included all relevant information for the EMA. Dr. Gül Erdemli (Novartis) led the efforts with contributions from consortium members, including Margarita Grammatikopoulou (CERTH), and Dr. Spiros Nikolopoulos (CERTH), co-lead of the project’s Work Package on RMTs, digital biomarkers and analytics and coordinator of the Interim Analysis activities.

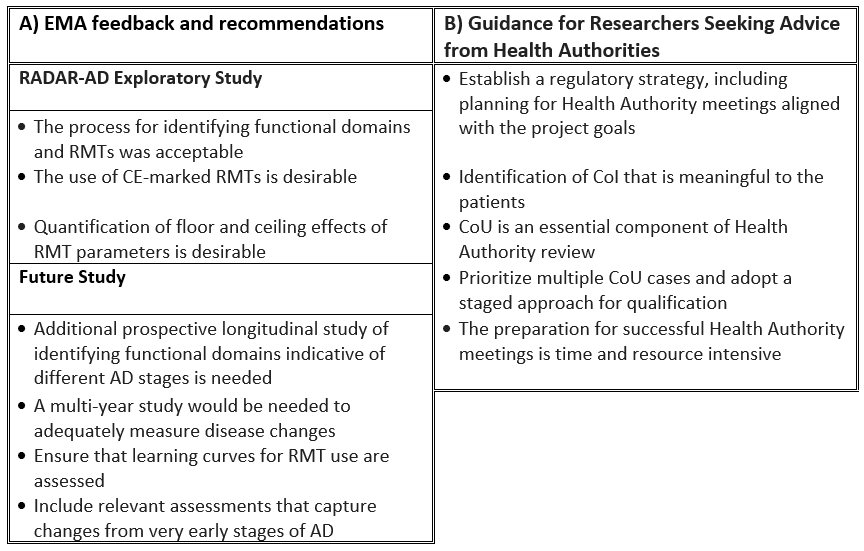
The recently published paper in npj Digital Medicine outlines the feedback received, along with key lessons learned to guide future projects (see Table 1). The consortium met with the EMA through the Innovation Task Force and Qualification Advice procedures, and discussed the project’s Concept of Interest (CoI), the Context of Use (CoU), the RMT selection process, the RADAR-AD clinical study design, and the results of the Interim Analysis, which included initial findings for 175 participants.
This research contributes to the growing body of evidence supporting the use of digital health technologies in clinical settings. The insights gained from the RADAR-AD project not only pave the way for future studies but also offer a roadmap for researchers and health authorities aiming to implement RMTs in clinical trials. As the demand for innovative assessment methods increases, the findings emphasize the importance of early regulatory engagement to ensure successful adoption of these technologies in AD research, clinical trials and drug development.
Read the full paper: G. Erdemli, M. Grammatikopoulou, B. Wagner, S. Vairavan, J. Curcic, D. Aarsland, G. Wittenberg, S. Nikolopoulos, M. Muurling, H. Froehlich, C. de Boer, N. M. Shanbhag, V. J. M. Nies, N. Coello, D. Gove, A. Diaz, S. Foy, W. Dartee & A.K. Brem , “Regulatory considerations for developing remote measurement technologies for Alzheimer’s disease research,” npj Digital Medicine 7, 232 (2024), September 2024.

